ChargePoint PharmaSafe® containment valve range relies on split butterfly valve technology to deliver small and large, highly potent powder transfers.
Our PharmaSafe valve offers numerous benefits including the reduced risk of contamination, meeting GMP and product quality requirements, maximising yield by transferring poorly flowing and high value products and removing costly secondary barrier containment and cumbersome PPE.
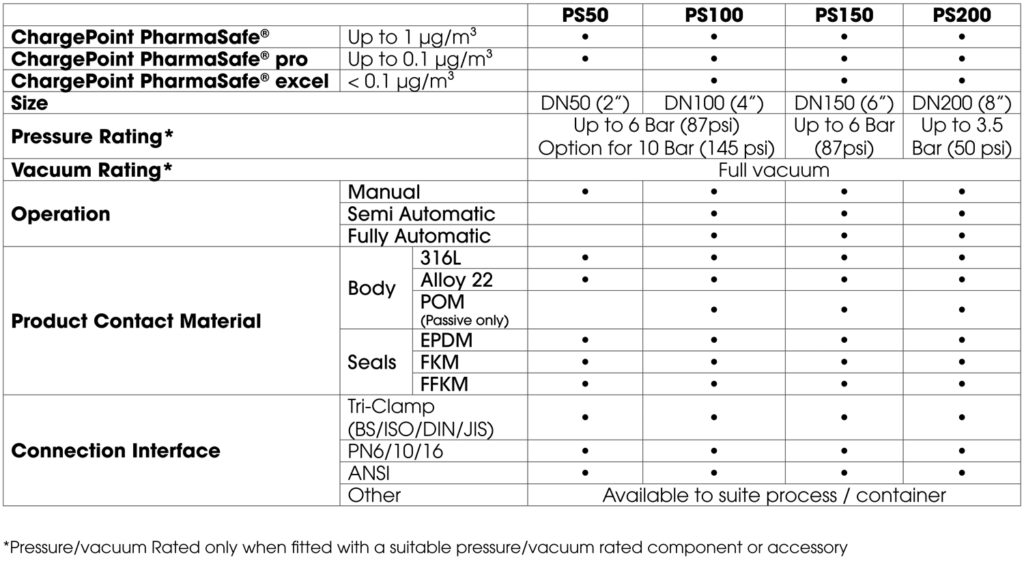
The three valves in the ChargePoint PharmaSafe® range include:
The containment performance of the ChargePoint PharmaSafe® valves has been independently validated by customers and third parties according to the ISPE SMEPAC (Standardised Measurement of Equipment Particulate Airborne Contamination) guideline.
The ChargePoint PharmaSafe® containment valve operates in the simple split butterfly valve sequence:
The ChargePoint PharmaSafe® range provides high containment powder transfers for all stages of the production process;