ChargePoint’s ChargeBag® PE-SBV is a single use bag for pharmaceutical ingredients that acts as a cost-effective and convenient container for the handling, storage and transfer of bio-pharmaceutical powders. The ChargeBag® PE-SBV reduces the risks associated with cross contamination and eliminate the time and expense associated with cleaning and validation.
Each ChargeBag® PE-SBV is 100% pressure decay tested to ensure integrity and supplied gamma sterilised ready to use in aseptic and many other cGMP manufacturing facilities. The bag has been comprehensively qualified according to the most rigorous standards for chemical, biological and physical compliance and delivers a well-rounded data profile characteristic of a wide range of products and process conditions.
The single use ChargeBag® PE-SBV is made with HiPureTM ULP7, ChargePoint’s pharma ready LLDPE film. HiPureTM ULP7 is manufactured with extremely high levels of integrity related to regulatory qualification, as well as robustness with excellent mechanical strength and anti-static properties providing both performance and purity for the safety of products and personnel.
The ChargeBag® PE-SBV comes with a ChargePoint single use passive split butterfly valve directly welded to the assembly, offering high levels of containment and sterility assurance performance.
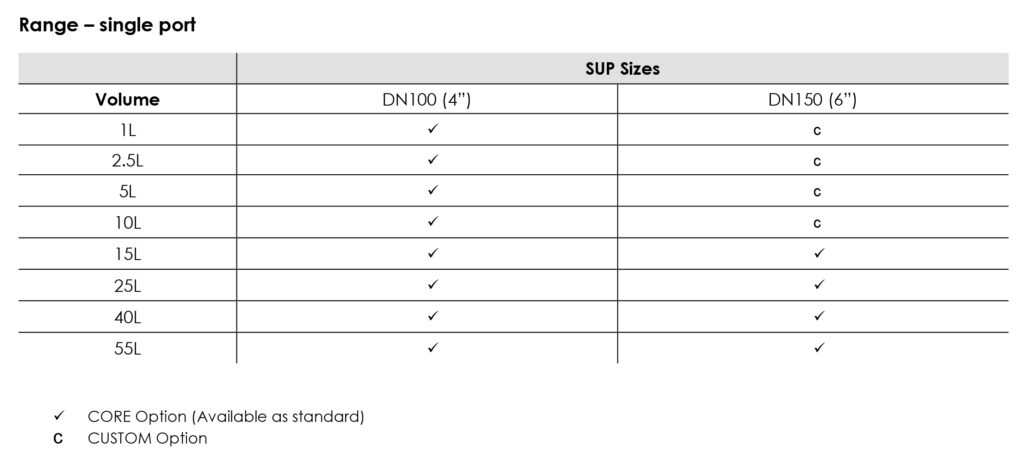
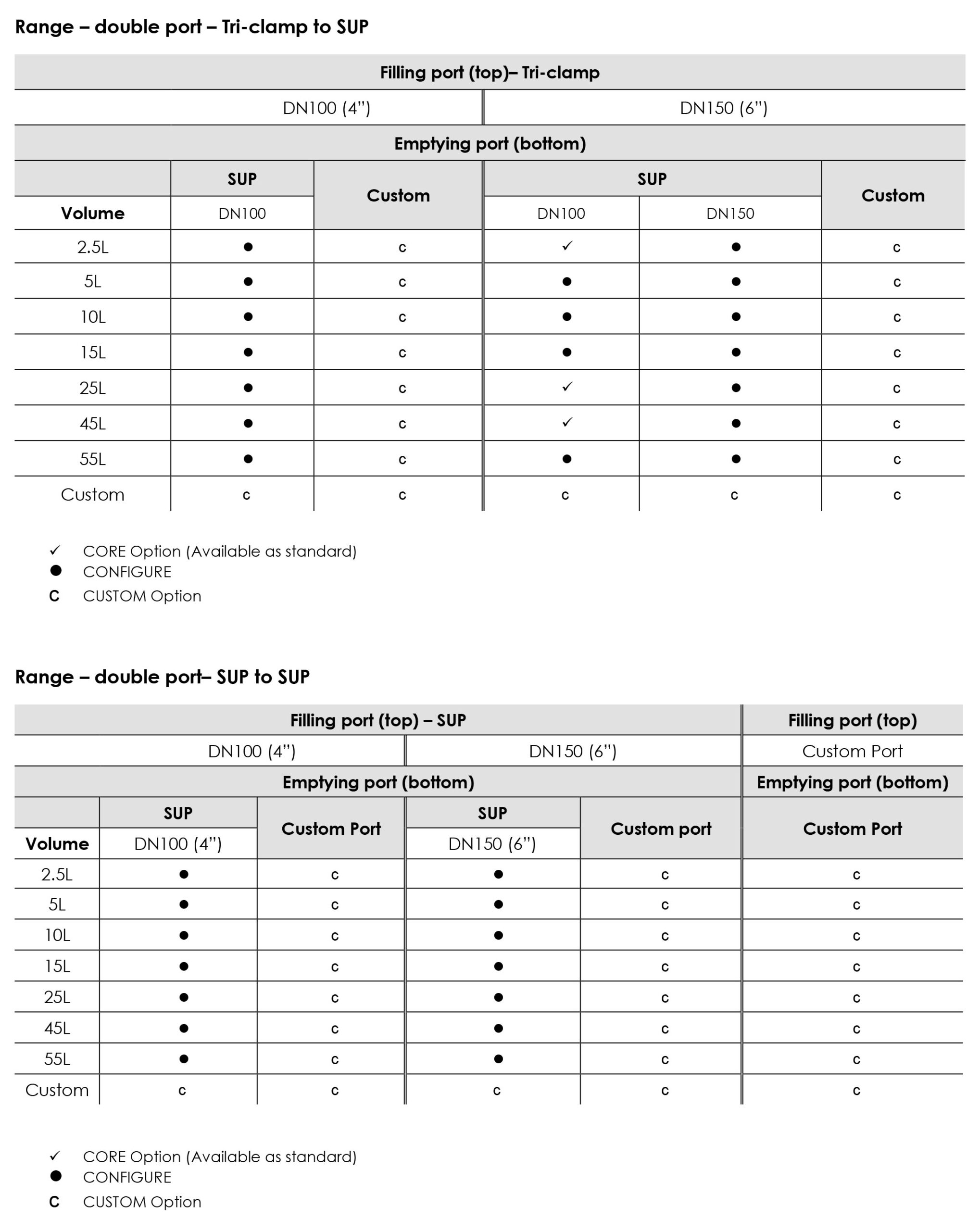
Available in single bottom port and also in a double port configuration.
A permanent, non-migrating, anti-static additive ensures that even cohesive powders can be recovered effectively from each transfer with minimal hold up. The pliable, transparent characteristics allow for physical manipulation of difficult powders and visual confirmation whilst maintaining the physical integrity of the bag.